
FNP TEAM4 project
Project team
Kenji Yamada (PI)
Kaichiro Endo (postdoc)
Toru Maeda (postdoc)
Shayan Sarkar (postdoc)
Alwine Wilkens (PhD student)
Mohamadreza Mirzaei (PhD student)
Karolina Małek (technician)
Collaborators
Prof. Mamiko Ozaki (Kobe University)
Prof. Ikuko Hara-Nishimura (Konan University)
Introduction
Plants have developed sophisticated defence systems to adapt to changes in the environment. Animal herbivores have the potential to damage plants seriously. Therefore, plants have evolved a defence system to alleviate them, which relies on species-specific metabolites. In the Brassicaceae plants, the defence metabolites are glucosinolates, which make spicy taste like wasabi and mustard, and it is a so-called “mustard oil bomb”. The glucosinolate biosynthesis pathway has evolved from the cyanogenic glucoside synthesis pathway in the plants of the order Brassicales. Many families of Brassicaceae share glucosinolates and cyanogenic glucosides, but Brassicaceae plants completely shifted to glucosinolate synthesis from cyanogenic glucoside synthesis. Glucosinolates are the precursor form of the active defence chemicals, and β-glucosidases (BGLUs) are required to activate these glucosides. Glucosinolates and BGLUs are separately stored, coming into contact and producing toxic molecules immediately against the cell damage by the herbivores, like a “mustard oil bomb”. Brassicaceae plants have two different sites for the storage of BGLUs; one is the vacuole of myrosin cells, and another is the endoplasmic reticulum (ER) bodies of epidermal cells (Figure 1). These variations of defence metabolites and enzyme storages raise the question of how Brassicaceae plants came to use these different defence systems; are there any benefits? An overview of the mustard oil bomb-based defence system is needed for further understanding the sophisticated defence system, which is essential for the survival of Brassicaceae plants.
Mutant seedlings lacking ER bodies are more sensitive to insects that normally would not attack living plants, indicating that the β-glucosidase in ER bodies is responsible for the defence against those insects. This finding is the first example showing that Arabidopsis thaliana has specific resistance mechanisms against certain insects in the seedlings. It is proposed that the interaction of plants and herbivores causes the divergence of defence strategies; the specific herbivores develop a tolerance to plant defence chemicals, and this will induce the development of new other defence chemicals in plants. However, there is no direct evidence the plant-herbivore interaction directs the divergence of plant defence chemicals and furthermore, it is still unknown about the impact of the changes in plant defence strategies on the behaviour of insects.
The aim of the project is to understand 1) How the plants have established chemical defence strategies and 2) How the herbivores respond and elude the plant defence. In this project, we collaborate with animal ethologists. Studies on the defence systems of plants and the feeding mechanism of animals have been performed independently. Despite much effort in each field, there is a lack of research on coevolutionary interaction between plants and herbivorous animals. Our collaboration between plant molecular biologist and animal neuroethologists will provide an innovative breakthrough to figure out the interactive development of their chemical strategies.
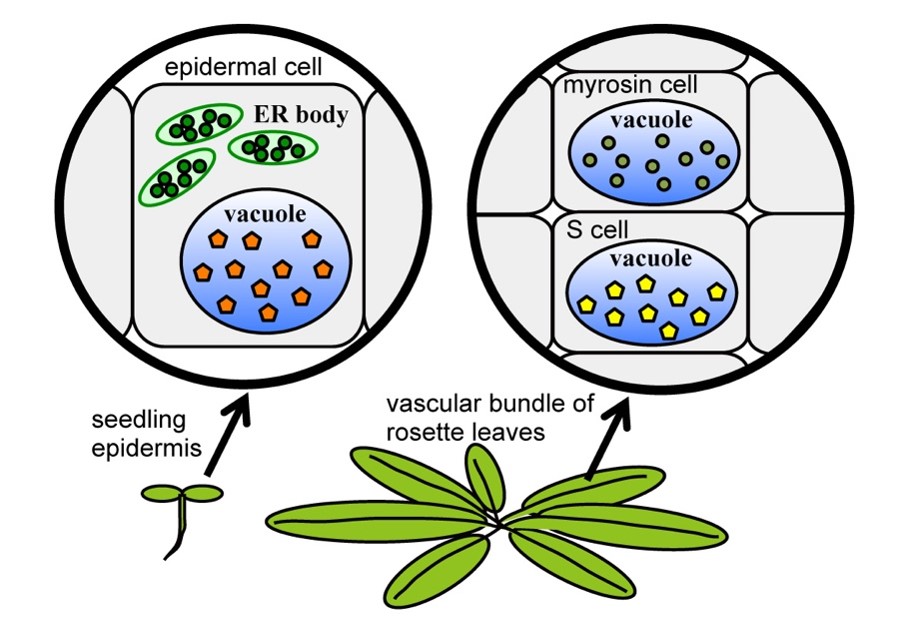
Figure 1. Two "mustard oil bomb" systems. A. thaliana seedlings have ER bodies in the epidermal cells. ER bodies accumulate β-glucosidase BGLU21/23 (green circle), and vacuoles accumulate the substrates (orange pentagon). In contrast, rosette leaves accumulate BGLU37/38 (yellow-green circle) in myrosin cells and glucosinolates (yellow circle) in S-cells. In both systems, glucosinolates and BGLUs come into contact and produce toxic molecules immediately against the cell damage by the herbivores.
Results
• Determination of NAI2 Homologues Function in the ER Body Formation
A. thaliana NAI2 is an ER body protein essential for ER body formation. We identified a NAI2 closest homologue, TSA1. We found that NAI2 gene is highly expressed in seedlings, whereas TSA1 gene expression is higher in the methyl jasmonate treated rosette leaves. The finding indicates that NAI2 is responsible for ER body formation in seedlings, and TSA1 is important for ER body formation in jasmonate-treated rosette leaves (Stefanik et al., 2019).
• Determination of Transcription Factors Regulating ER Body Formation
NAI1/bHLH020 regulates the expression of genes encoding proteins in cER body components, such as NAI2 and PYK10. We found that NAI1 binds to the G-box cis-regulatory elements of the NAI2 promoter (Sarkar et al., 2020). We found two closest homologues, MYB47 and MYB95, co-express with BGLU18 and TSA1 genes and reduced BGLU18 and TSA1 gene expression in the jasmonate-treated myb47 myb95 double mutant leaves. The finding suggests that MYB47 and MYB95 regulate ER body formation in the jasmonate signalling pathway.
• Involvement of Giant Cell Differentiation in Leaf ER Body Formation
We found that specific types of cells (marginal cells, epidermal cells covering the midrib and giant pavement cells) constitutively produce ER bodies in rosette leaves (Nakazaki et al., 2019; Nakazaki et al., 2019), and these cells accumulate PYK10 and BGLU18 in their ER bodies. We found that the expression of NAI1, NAI2, and PYK10 was reduced in the mutants lacking giant cells, suggesting that giant cell differentiation is involved in the ER body formation in rosette leaves.
• Fly Behavior Analysis against ER Body-Deficient Plants
We found that isothiocyanates from Arabidopsis seedlings stimulate both the olfactory and gustatory receptor organs of blowflies (Phormia regina). The blowfly feeding motivation was suppressed by the odour and taste of the wild type but not by those of myrosinase or glucosinolate-deficient mutants. The ancestral plant defence chemical, hydrogen cyanide, stimulated none of the olfactory and gustatory receptor organs of blowflies but reduced their survival rates. Hence, Brassicaceae plants appear to have evolved a ‘win–win’ defence strategy to reduce the feeding motivation of insect herbivores, thereby minimising fatal damage to both plants and insects.
Budget
• 2 973 006,00 PLN
• Contribution from the European Regional Development Fund: 100%
• Contract number: POIR.04.04.00-00-41CA/17-00
Publications
• 1. Sarkar S, Stefanik N, Kunieda T, Hara-Nishimura I, Yamada K. (2021). The Arabidopsis transcription factor NAI1 activates the NAI2 promoter by binding to the G-box motifs. Plant Signal. Behav. 16, e1846928.
• 2. Basak AK, Mirzaei M, Strzałka K, Yamada K. (2021). Texture feature extraction from microscope images enables a robust estimation of ER body phenotype in Arabidopsis. Plant Methods 17, 109.
• 3. Stefanik N, Bizan J, Wilkens A, Tarnawska-Glatt K, Goto-Yamada S, Strzałka K, Nishimura M, Hara-Nishimura I, Yamada K. (2020). NAI2 and TSA1 drive differentiation of constitutive and inducible ER body formation in Brassicaceae. Plant Cell Physiol. 61, 722-734.
• 4. Nakazaki A, Yamada K, Kunieda T, Tamura K, Hara-Nishimura I, Shimada T. (2019). Biogenesis of leaf endiolasmic reticulum body is regulated by both jasmonate-dependent and independent pathways. Plant Signal. Behav. 14, e1622982.
• 5. Nakazaki A, Yamada K, Kunieda T, Sugiyama R, Hirai YM, Tamura K, Hara-Nishimura I, Shimada T. (2019). Leaf endoplasmic reticulum bodies identified in Arabidopsis rosette leaves are involved in defense against herbivory. Plant Physiol. 179, 1515-1524.
Patents
• 1. Sarkar S, Basak AK, Yamada K, Bizan J, Czerniawski P, Bednarek P. A promoter activated by the MYB47 and MYB95 proteins and the expression system. Application number, P.438703, August 6, 2021, Poland
• 2. Sarkar S, Yamada K, Stefanik N, Nishimura I, Kunieda T. A promoter activated by the NAI1 protein and the expression system containing it. NR242142, December 9, 2020, Poland.



