
Our three main areas of research are; photobiology, in particular the impact of short wavelength radiation on plant physiology, issues related to the interaction of light with plant leaves and the development of software tools for Atomic Force Microscopy (AFM).
The main area of our research is photobiology, in particular the impact of short wavelength radiation on plant physiology. Our studies concentrate on UV/blue light photoreceptors called phototropins. These proteins contain two flavin mononucleotides as chromophores in the photo-sensory LOV (Light, Oxygen, and Voltage) domains at the N-terminus and a Serine/Threonine kinase domain at the C-terminus. Two phototropins (phototropin1 and phototropin2) are encoded in the genome of the model plant Arabidopsis thaliana. They share highly redundant functions, which fine-tune photosynthesis, including chloroplast movements, phototropism, stomatal opening, leaf positioning, and blade formation. Phototropins show different photosensitivity and can change their signalling outcomes dependent on the light intensity. For instance, low light induces the chloroplast accumulation response, which is controlled by both phototropin1 and phototropin2. High light elicits chloroplast avoidance, mediated by phototropin2. Phototropin1 triggers only a residual avoidance response. The molecular basis for the difference in the signalling pathways leading to chloroplast movements in low and high light remains poorly understood. More downstream signalling components, such as proteins phosphorylated by phototropins, are unknown. Phototropin ability to change the pathways from chloroplast accumulation to avoidance may stem from differences in their structures. Moreover, phototropin dimerization seems to modify their signalling outcomes. Currently, we focus on molecular aspects of photoreceptor functioning to determine how phototropins control chloroplast movements. We also investigate ecologically relevant aspects of chloroplast movements, such as the impact of UV radiation. We would like to determine the importance of chloroplast movements in the natural environment.
The second topic investigated by our group is the interaction of light with plant leaves. We specialize in designing devices build of stock components to measure leaf properties, such as transmittance and reflectance. Our equipment is operated by our open-source software, BeamJ. Our ray tracing tool is available at https://github.com/plantPhotobiologyLab/openRayTracer.
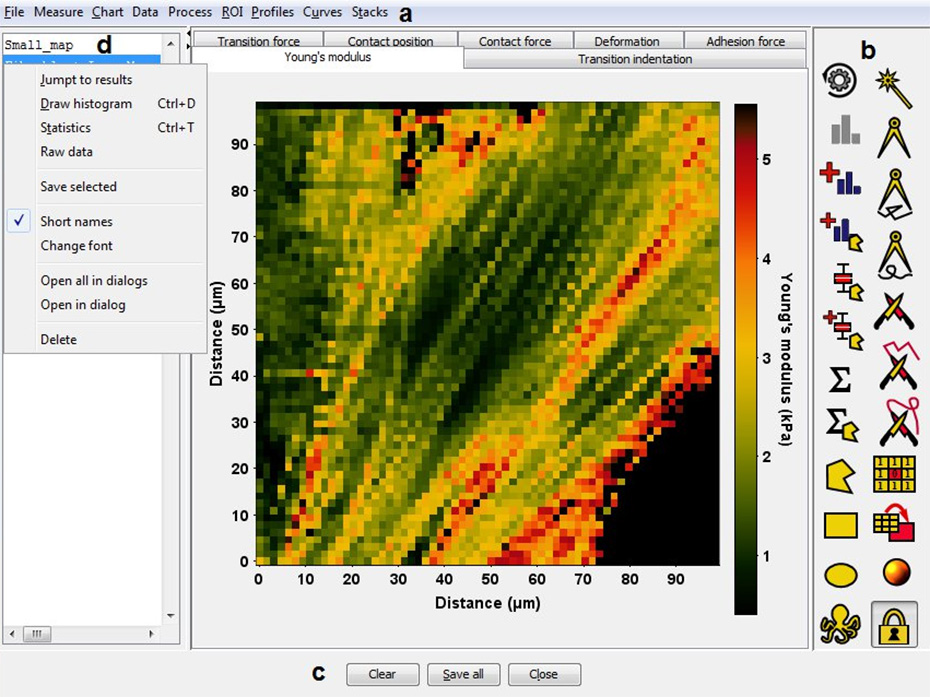
The third area of research is devoted to the development of software tools for Atomic Force Microscopy (AFM). AtomicJ, created and still actively developed by Paweł Hermanowicz, is an application for the analysis of images and force-distance curves recorded with AFM. It allows for parallel processing of force–volume measurements, generating maps of mechanical properties of the sample, in particular maps of Young’s modulus, adhesion force, transition indentation, sample height, and deformation. AtomicJ supports several models of contact between the AFM tip and the sample, including models suitable for blunt tips and thin samples, models of adhesive contact, and models for hyper-elastic materials. Together with the source code, AtomicJ is distributed through SourceForge, under the GPL licence. Further information at https://github.com/pawelHerm/AtomicJ
Laboratory of Photobiology is responsible for the Laser Scanning Confocal Microscope available at MCB. The person taking care of the microscope, troubleshooting and dealing with service issues is Dr. Paweł Hermanowicz. The microscope is equipped with a confocal unit and the AiryScan Detector, improving resolution up to 140 nm, also in the Z – direction. The microscope can be used to perform lambda stacking, which consists of simultaneous recording of 32 images, each corresponding to less than 10 nm of the emission spectrum, to separate fluorophores with partly overlapping emission spectra. Imaging of live samples for multiple hours is possible thanks to the incubation system, which allows for control of temperature, CO2 and oxygen concentration, as well as the definite focus system, reducing focal shift. New users are welcome to use the microscope after obtaining the training (3 h). The fees for academic users are low. The same preferential fee is offered to all users from the university units participating in POB BIOS.

dr Justyna Łabuz
Read More o dr Justyna ŁabuzŁabuz Justyna, PhD, Group Leader, justyna.sojka@uj.edu.pl, (+48 12) 664 6109
Hermanowicz Paweł, PhD, pawel.hermanowicz@uj.edu.pl, (+48 12) 664 6109
Anna Hebda, PhD, DSc., a.hebda@uj.edu.pl, (+48 12) 664 6109
Anna Kozłowska-Mroczek, MSc, Eng., anna.1.kozlowska@uj.edu.pl, (+48 12) 664 6109
Aleksandra Giza, MSc, a.giza@doctoral.uj.edu.pl, (+48 12) 664 6109
Aneta Prochwicz, MSc, aneta.prochwicz@doctoral.uj.edu.pl, , (+48 12) 664 6109

2022 – 2026 OPUS 21, 2021/41/B/NZ1/02826 from the National Science Centre (Poland) “How does phototropin2 control chloroplast positioning in Arabidopsis?” PI: Justyna Łabuz
2021 – 2023 POB BIOS, Jagiellonian University, “The impact of chloroplast avoidance on photosynthetic efficiency of plants in fluctuating light” PI: Justyna Łabuz
2021 – 2022 POB BIOS, Jagiellonian University, “The role of ultraviolet radiation in colonization of plants by endophytes”, PI: Justyna Łabuz
2021 – 2022 MINIATURA 2020/04/X/NZ4/01256, from the National Science Centre (Poland) “Application of polarized light to improve sensitivity of the method of detection of chloroplast movements based on leaf reflectance measurements”, PI: Paweł Hermanowicz
2018 – 2022 OPUS 13, 2017/25/B/NZ3/01080 from the National Science Centre (Poland) “Dissecting the molecular basis of phototropin signaling to chloroplast movements in Arabidopsis thaliana”, PI: Justyna Łabuz
Łabuz J, Sztatelman O, Hermanowicz P. 2022. Molecular insights into the phototropin control of chloroplast movements, Journal of Experimental Botany, erac271, https://doi.org/10.1093/jxb/erac271
Łabuz J, Sztatelman O, Jagiełło-Flasińska D, Hermanowicz P, Bażant A, Banaś AK, Bartnicki F, Giza A, Kozłowska A, Lasok H, Krzeszowiec-Jeleń W, Gabryś H, Strzałka W. 2021. Phototropin interactions with SUMO proteins, Plant and Cell Physiology 62, 693–707.
Eckstein A, Grzyb J, Hermanowicz P, Zgłobicki P, Łabuz J, Strzałka W, Dziga D, Banaś AK. 2021. Arabidopsis phototropins participate in the regulation of dark-induced leaf senescence. International Journal of Molecular Sciences 22, 1836.
Hermanowicz P. 2021. Determination of Young’s modulus of samples of arbitrary thickness from force distance curves: numerical investigations and simple approximate formulae. International Journal of Mechanical Sciences193, 106138.
Hermanowicz P, Banaś AK,. Sztatelman O, Gabryś H, Łabuz J. 2019. UV-B Induces Chloroplast Movements in a Phototropin-Dependent Manner. Frontiers in Plant Science, 10, 1279.
Eckstein A, Grzyb J, Hermanowicz P, Łabuz J, Banaś AK. 2019. A role for GLABRA1 in dark-induced senescence. Acta Biochimica Polonica, 66, 243–248.
Hart JE, Sullivan S, Hermanowicz P, Petersen J, Diaz-Ramos LA, Hoey DJ, Łabuz J, Christie JM. 2019. Engineering the phototropin photocycle improves photoreceptor performance and plant biomass production. PNAS, 116, 12550-12557.
Robson TM, Aphalo PJ, Banaś AK, Barnes PW, Brelsford CC, Jenkins GI, Kotilainen TK, Łabuz J, Martínez-Abaigar J, Morales LO, Neugart S, Pieristè M, Rai N, Vandenbussche F, Jansen MAK. 2019. A perspective on ecologically relevant plant-UV research and its practical application. Photochemical and Photobiological Sciences, 18, 970-988.
Kowalska E, Bartnicki F, Fujisawa R, Bonarek P, Hermanowicz P, Tsurimoto T, Muszynska K, Strzalka W. 2018. Inhibition of DNA replication by an anti-PCNA aptamer/PCNA complex. Nucleic Acids Research, 46, 25-41.
Grzyb J, Gieczewska K, Łabuz J, Sztatelman O. 2018. Detailed characterization of Synechocystis PCC 6803 ferredoxin:NADP+ oxidoreductase interaction with model membranes. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1860, 281-291.
Banaś AK, Hermanowicz P, Sztatelman O, Łabuz J, Aggarwal Ch, Zgłobicki P, Jagiełło-Flasińska D, Strzałka W. 2018. 6,4 - PP Photolyase Encoded by AtUVR3 is Localized in Nuclei, Chloroplasts and Mitochondria and Its Expression is Down-Regulated by Light in a Photosynthesis-Dependent Manner. Plant and Cell Physiology, 59, 44–57.
Gabryś H, Banaś AK, Hermanowicz P, Krzeszowiec W, Leśniewski S, Łabuz J, Sztatelman O. 2017. Photometric Assays for Chloroplast Movement Responses to Blue Light. Bio-protocol, 7:e2310.
Sztatelman O, Łabuz J, Hermanowicz P, Banaś AK, Bażant A, Zgłobicki P, Aggarwal C, Nadzieja M, Krzeszowiec W, Strzałka W, Gabryś H. 2016. Fine tuning chloroplast movements through physical interactions between phototropins. Journal of Experimental Botany, 67, 4963-4978.
Łabuz J, Samardakiewicz S, Hermanowicz P, Wyroba E, Pilarska M, Gabryś H. 2016. Blue light-dependent changes in loosely bound calcium in Arabidopsis mesophyll cells: an X-ray microanalysis study. Journal of Experimental Botany, 67, 3953-3964.

