The main interest of our group is the molecular (patho)mechanisms leading to endothelial cell dysfunction in various human disease entities. Endothelial cells, lining the inner wall of blood vessels, are present in all tissues and organs, and their proper functioning is essential for maintaining body homeostasis.
The major interest of our group is connected with molecular mechanisms that lead to endothelial dysfunction in different human diseases. Endothelial cells are present in all tissues and organs and their proper function is essential for keeping the homeostasis of the whole individual. By dissecting the molecular mechanisms involved in the pathophysiology of these cells, new therapeutic targets could be selected in the future.
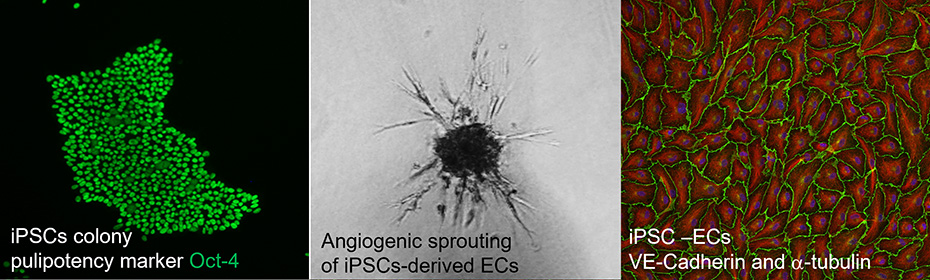
Currently, we are looking at the molecular background of endothelial dysfunction, observed in patients with maturity onset diabetes of the young (MODY), which is a monogenic diabetic disease. Our research is based on the usage of induced pluripotent stem cells (iPSCs) lines modified by CRISPR/Cas9 gene editing thus creating precise disease-relevant modeling tool.
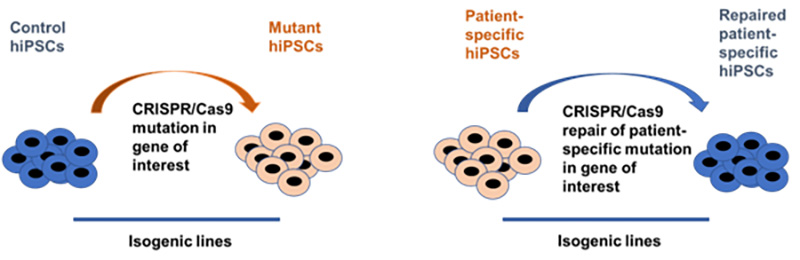
Using this disease modeling system, we found that cells with heterozygous mutation in HNF1A gene have increased vascular permeability after stimulation with pro-inflammatory cytokine (Kachamakova-Trojanowska et al., 2019). And this effect was even more pronounced in the cells with biallelic mutation. Currently, we try to dissect the molecular background of the observed increase of the vascular permeability and to reveal its possible relation to HNF1A mutation.
- Derivation of iPSCs from human somatic cells
- Differentiation of iPSCs toward endothelial cells
- CRISPR/Cas9 gene editing techniques
- Western blot
- Immunostaining
- Culture of cells under flow conditions (ibidi pump system)
- Live imaging (Nanolive)
- Gene expression
- Flow cytometry analysis
- Cell sorting techniques

Neli Kachamakova-Trojanowska, PhD
Read More o Neli Kachamakova-Trojanowska, PhD
Dawid Skoczek, MSc – PhD student
Research technician
Ms. Dominika CzekalskaAlumni members:
dr Damian Kloska
dr Marian Babincak
Publications
- Skoczek et al., Maturity Onset Diabetes of the Young-New Approaches for Disease Modelling, Int J Mol Sci. 2021. https://www.mdpi.com/1422-0067/22/14/7553
- Kachamakova-Trojanowska et al., HIF-1 stabilization exerts anticancer effects in breast cancer cells in vitro and in vivo. Biochem Pharmacol. 2020 https://www.sciencedirect.com/science/article/pii/S0006295220301507?via%3Dihub
- Białopiotrowicz et al., Serine Biosynthesis Pathway Supports MYC-miR-494-EZH2 Feed-Forward Circuit Necessary to Maintain Metabolic and Epigenetic Reprogramming of Burkitt Lymphoma Cells. Cancers (Basel). 2020 https://www.mdpi.com/2072-6694/12/3/580
- Szade et al., Heme oxygenase-1 deficiency triggers exhaustion of hematopoietic stem cells. EMBO Rep. 2020 https://www.embopress.org/doi/full/10.15252/embr.201947895
- Rozga et al., Novel engineered TRAIL-based chimeric protein strongly inhibits tumor growth and bypasses TRAIL resistance. Int J Cancer. 2019 https://onlinelibrary.wiley.com/doi/10.1002/ijc.32845
- Kachamakova-Trojanowska et al., Human iPSCs-Derived Endothelial Cells with Mutation in HNF1A as a Model of Maturity-Onset Diabetes of the Young, Cells. 2019 https://www.mdpi.com/2073-4409/8/11/1440
- Szade et al., Cobalt protoporphyrin IX increases endogenous G-CSF and mobilizes HSC and granulocytes to the blood. EMBO Mol Med. 2019 https://www.embopress.org/doi/full/10.15252/emmm.201809571
- Kachamakova-Trojanowska et al., Molecular profiling of regulatory T cells in pulmonary sarcoidosis. J Autoimmun. 2018 https://www.sciencedirect.com/science/article/pii/S0896841118302373?via%3Dihub
- Stepniewski et al., Heme oxygenase-1 affects generation and spontaneous cardiac differentiation of induced pluripotent stem cells. IUBMB Life. 2018 https://iubmb.onlinelibrary.wiley.com/doi/10.1002/iub.1711
- Stepniewski et al., Induced pluripotent stem cells as a model for diabetes investigation. Sci Rep. 2015 https://www.nature.com/articles/srep08597
-
2022- 2026 COST action CA21113 Genome Editing to Treat Humans Diseases (GenE-Humdi) – management committee participant Dr hab. Neli Kachamakova-Trojanowska
2021-2025 Project Sonata-Bis headed by Dr. Neli Kachamakova-Trojanowska: “Molecular background of microvascular complications in HNF1A-MODY patients: induced pluripotent stem cells as disease modeling tool”
2017-2021 Project OPUS headed by Dr. Neli Kachamakova-Trojanowska:“Editing of HNF1A gene in human induced pluripotent stem cells with CRISPR/Cas9 for disease modeling of endothelial (dys)function in maturity onset diabetes of the young (MODY)”
Neli Kachamakova-Trojanowska, PhD (neli.kachamakova-trojanowska@uj.edu.pl).
-
We are looking for a motivated postdoctoral researcher who is curious about molecular mechanisms of endothelial dysfunction, related to maturity onset diabetes of the young. If you have experience in molecular and cellular biology or metabolomics, an excellent command in English, can-do attitude and fast learning abilities, please follow the link to the application: https://mcb.uj.edu.pl/oferty-pracy/-/journal_content/56_INSTANCE_WbR3ltHEfYue/150912362/155027343
Application deadline: 31.01.2024

Leader | Monika Jakubowska, PhD
phone: 12 664 6468; room: 2/24

Kinga Stopa, MSc

Daria Krszysztofik, MSc
Funding | Grants
2022-2024 NCN Project PRELUDIUM 20, awarded to Daria Krzysztofik: "Long-term exposure to high copper: does it affect the production of pancreatic digestive enzymes?"
2021-2022 POB BIOS Minigrant awarded to Dr. Monika Jakubowska
Title: "Copper misbalance and impaired exocrine pancreatic functions - ex vivo analyses of the Atp7b toxic milk mouse model"
2018-2020 FNP Project HOMING headed by Dr. Monika Jakubowska:“Targeting mechanisms of pancreatic cancer resistance against therapy: specific role of tumor microenvironment”
Selected publication
Kusiak et al., Activation of pancreatic stellate cells attenuates intracellular Ca2+ signals due to downregulation of TRPA1 and protects against cell death induced by alcohol metabolites, Cell death dis. 2022.
Ferdek et al., When healing turns into killing - the pathophysiology of pancreatic and hepatic fibrosis, J Physiol. 2022.
Du et al., A microRNA checkpoint for Ca2+ signaling and overload in acute pancreatitis, Mol Ther. 2022
Jakubowska et al., Electron paramagnetic resonance spectroscopy reveals alterations in the redox state of endogenous copper and iron complexes in photodynamic stress-induced ischemic mouse liver Redox Biol. 2020.
Stopa et al., Pancreatic Cancer and Its Microenvironment-Recent Advances and Current Controversies. Int J Mol Sci. 2020
Kusiak et al., Signaling in the Physiology and Pathophysiology of Pancreatic Stellate Cells - a Brief Review of Recent Advances, Front Physiol. 2020.
Jakubowska et al., ABT-199 (Venetoclax), a BH3-mimetic Bcl-2 inhibitor, does not cause Ca 2+ -signalling dysregulation or toxicity in pancreatic acinar cells. Br J Pharmacol 2019
Vervliet et al., BH4 domain peptides derived from Bcl-2/Bcl-XL as novel tools against acute pancreatitis, Cell Death Discov. 2018
Ferdek et al., BH3 mimetic-elicited Ca 2+ signals in pancreatic acinar cells are dependent on Bax and can be reduced by Ca 2+-like peptides, Cell Death Dis. 2017.

