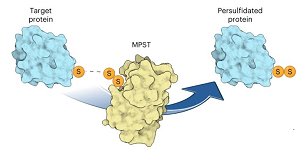
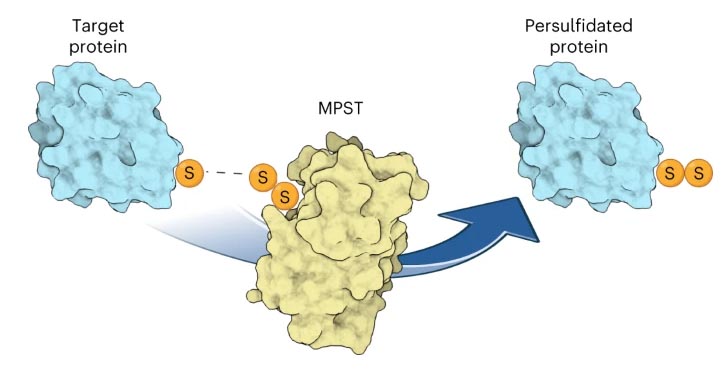
Protein S-persulfidation (P-SSH) is recognized as a common posttranslational modification. It occurs under basal conditions and is often observed to be elevated under stress conditions. However, the mechanism(s) by which proteins are persulfidated inside cells have remained unclear.
Very recent work from University of Heidelberg in collaboration with the Max Planck Reseaerch Gorup at the Malopolska Centre of Biotechnology (MCB/UJ) shows how this small chemical modification can be installed by a direct enzymatic reaction. In detail, the work identified 3-mercaptopyruvate sulfurtransferase (MPST) as one of the key enzymes in generating and maintaining protein persulfides in human cells. The ground-breaking results were recently published in “Nature Chemical Biology”
Researchers from the Max Planck Research Group at the MCB of the Jagiellonian University in Krakow (Poland) contributed their recently established tools and expertise to the study from the Dick group at the DKFZ in Heidelberg, Germany. The team experimentally confirmed the hypotheses that protein persulfides can be generated from an enzymatically-driven process. This is surprising, taking into account how enzymes facilitate and speed up chemical reactions. It was known that MPST takes away the sulfur from 3-mercaptopyruvate, a product of cysteine transamination, generating a persulfide on the active site cysteine of MPST. Nonetheless, the published study now clearly shows that this persulfidated MPST then transfers the persulfide outer sulfur to other substrates, including protein thiols. Foremost, the authors show that depleting MPST levels in human cells also reduced overall levels of protein persulfidation. Vice versa, increasing MPST levels induced cellular protein persulfidation levels. These results convincingly show that MPST is a key in protein persulfidation in our cells.
The team in Krakow headed by Sebastian Glatt recently also revealed that a downstream target of MPST, namely Urm1, can also transfer sulfur from its thiocarboxylated C-terminus onto cysteines in certain target proteins. The combined results of the studies have defined a novel pathway to post-translationally modify proteins and their enzymatic function, which are highly relevant for protecting ourselves against oxidative stressors and to the process of ageing.
The work was supported by funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 101001394, PI Sebastian Glatt).
MPST is able to deliver sulfur to certain tRNAs and proteins. The Urm1-mediated tRNA thiolation requires additional downstream enzymes, but the persulfidation of cysteines by Urm1 is directly catalysed by an UBL-like conjugation reaction that is triggered by oxidative stress.